
Direct lithium extraction (DLE) from brine1
Introduction
Produced water is the largest byproduct of oil and gas production2. It is produced when subsurface brine is separated from hydrocarbons3. Oil and gas field-produced water (OGPW) contains cations such as lithium and strontium which are of significant environmental concern2. Lithium may cause oxidative stress, inhibition of growth and reproduction, hepatotoxicity, and neurological disorders in aquatic life. Most OGPW are disposed by injected them into underground wells24.
Lithium has various uses. It is used in batteries, lubricants, glassware, metallurgy, ceramics, and chemicals/pharmaceuticals5. Between 2010 and 2021, the global use of lithium rose from 20,000 to 98,000 tons. it is estimated that worldwide consumption of lithium will grow from 37 kilotons to 0.38 megatons from 2016 to 20286. Furthermore, it is estimated that global lithium supply may reach a gap of nearly 50% by 20303. It is therefore important to look for new approaches in lithium production.
One way to produce lithium is to extract it from OGPW5. Recovery processes include adsorption/ion exchange, solvent extraction, precipitation/evaporation, and membrane separation. Membrane separation has been amongst the most studied lithium recovery process due to better energetics and lower footprint7. In Canada, MGX Minerals Inc. has utilized a membrane separation technique called nanofiltration (NF) in the Sturgeon Lake oil field which reduces lithium production time from 18 months to under 1 day, amounting to a 99% reduction in processing time73. With the advent of novel membrane separation technologies, lithium extraction from OGPW has never been more viable.
Canada’s two main oil/gas fields are the Williston Basin and the Alberta Basin3. The Williston Basin spans from southern Saskatchewan to southwestern Manitoba, while the Alberta Basin is mainly located in Alberta, with some parts in British Columbia. Alberta’s oilfield brines were identified to have an elevated concentration of lithium relative to seawater and freshwater4. Rather than disposing OGPW in underground wells, there may be economic incentive in extracting lithium from OGPW.
One of the biggest hurdle in applying lithium extraction technologies into a larger scale is that its economic viability largely depends on the lithium content of OGPW and the market price of lithium5. Regulatory policies can be used to enforce adoption of these technologies, one of the most straightforward being environmental policies. Knowing the health risks of lithium in OGPW, using environmental policies to enforce lithium extraction may not only make OGPW safer for the environment but also incentivize oil and gas industries to contribute to the global lithium supply.
Purpose
The purpose of this research is to look for available legal frameworks that can be used to enforce lithium extraction from oil and gas field-produced water. As noted in the introduction section, one of the biggest hurdle in applying lithium extraction technologies is the market price of lithium. Using environmental policies can reap double benefits; its primary benefit being environmental protection since lithium-free OGPW is now being injected into underground wells. Its secondary benefit is that it enforces oil and gas industries to produce its own lithium, which helps relieve global lithium supply constraints.
Rationale
This research is worth doing because of the predicted global lithium supply shortage in the future. Hopefully this research can be used as a template for adoption of similar policies so that Canada can be a major player in lithium production.
Enabling Acts and Regulations
The main regulatory tool to manage the injection/disposal of produced water into disposal wells is Directive 051: Injection and Disposal Wells - Well Classifications, Completions, Logging, and Testing Requirements8. This directive is issued by the Alberta Energy Regulator (AER) and is enabled by Alberta’s Oil and Gas Conservation Act since management of minerals and non-renewables fall under provincial powers9. The AER is a quasi-independent government agency that is responsible for providing “efficient, safe, orderly and environmentally responsible development of energy resources in Alberta”. It has authority over the licensing of wells for the disposal of produced water.
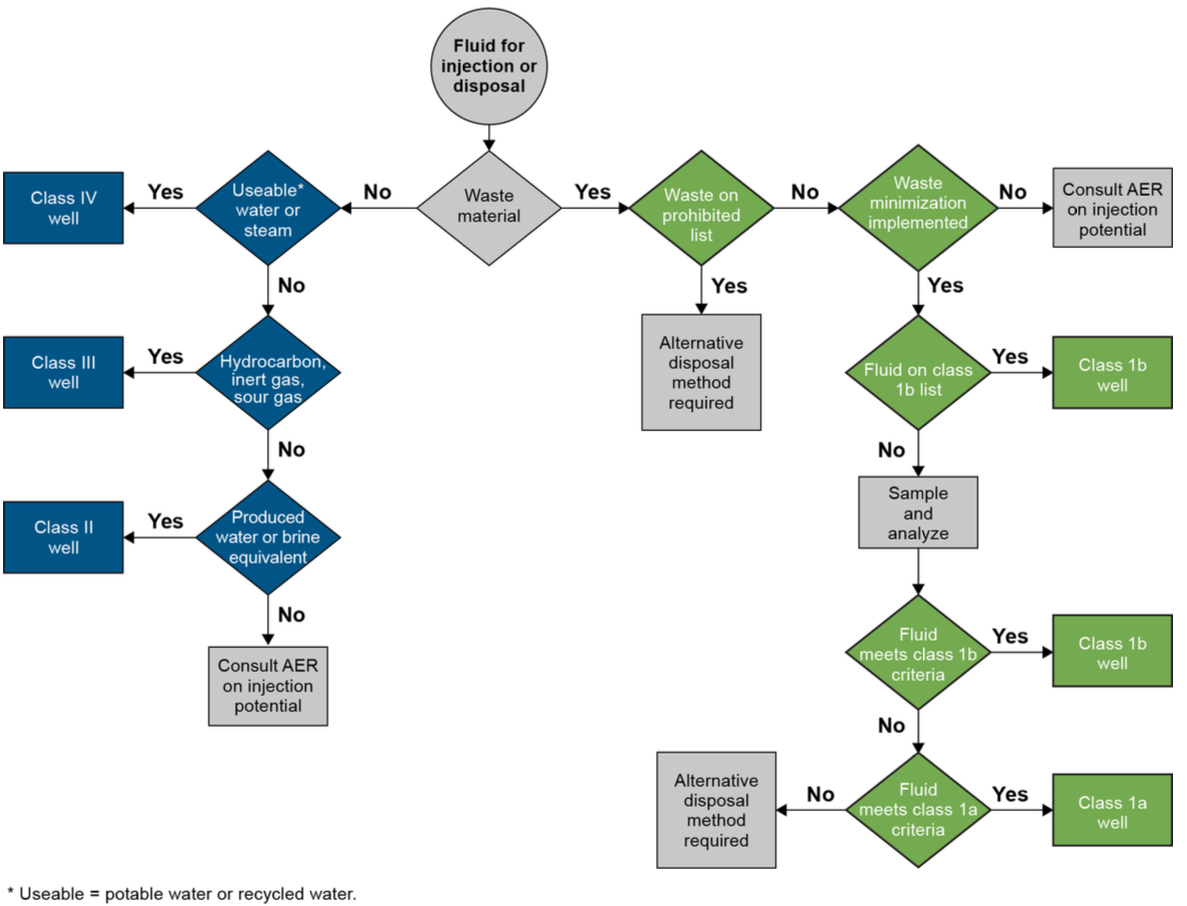
Well classification decision tree.8
Wells that are used to dispose produced water are licensed as Class II wells8. There are no specified limits on constituent concentrations or water quality parameters for the produced water that is injected into the well. This is contradictory to the principles of the directive which is to implement waste minimization. Wells licensed for the disposal of waste fluids with metal concentrations below a specified concentration limit fall under Class 1a and Class 1b, however there is no limit for lithium. Waste fluids are not permitted to be diluted to meet metal concentration limits.
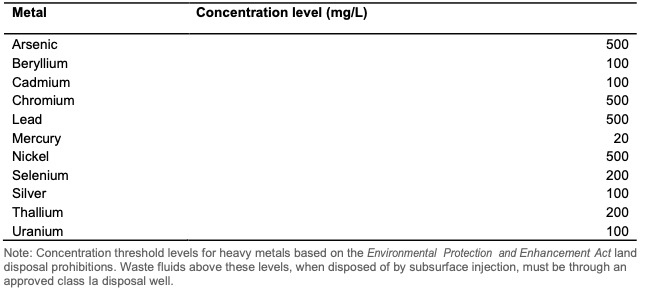
Metal concentration limits for the disposal of waste fluid8
Proposed Changes
I think Class II well licenses should be discontinued or amended. Disposal of produced water should follow the same requirements as waste fluids (Class 1a and Class 1b), and the metal concentration limits should be modified to include lithium with a low concentration limit. This should force oil and gas production companies to minimize wasting of lithium by preventing it from being disposed in underground wells.
Limiting Transport of Lithium in Produced Water Through Amendments to the Transportation of Dangerous Goods Regulations
Produced water can be transported and injected into a well at a different location as a means of disposal or to enhance oil recovery10. The transport of produced water in highway tanks is subject to the Excepted Quantities Exemption of the Transportation of Dangerous Goods (TDG) regulations, but only if it is composed of constituents listed in Schedule 3 of the TDG regulations. Therefore, produced water needs to be tested to quantify the amount of constituents such as strontium and lithium to see whether it is subject to TDG regulations11. Schedule 1 of the TDG Regulations provides the excepted quantities of various forms of lithium12. The excepted quantities are listed in total volume (mL), which makes it difficult to quantify the volume of lithium when it is suspended or dissolved in produced water. I propose an amendment to the TDG regulations, where a new class for constituents in produced water is added, and the excepted quantities are listed as a concentration measurement with low maximum values. Limiting the transport of lithium in produced water will hopefully force oil and gas exploration and production companies to utilize in-situ lithium extraction technologies to prevent lithium from being wasted.
Footnotes
-
Parker, S., Franklin, B., Williams, A., Cohen, B., Clifford, M., & Rohde, M. (2022). Potential Lithium Extraction in the United States: Environmental, Economic, and Policy Implications (https://www.scienceforconservation.org/products/lithium). ↩
-
Talebi, M., Rezaei, A., & Rafiei, Y. (2025). Application of sodium carbonate and sodium sulfate for removal of lithium and strontium from oilfield produced water. Scientific Reports, 15(1), 18895. https://doi.org/10.1038/s41598-025-04074-5 ↩ ↩2 ↩3
-
Zhang, C., Liu, B., He, S., Chen, J., & Sun, T. (2025). Associated lithium extraction from oil/gas field produced water: Resources, technologies, and practices. Journal of Water Process Engineering, 77, 108356. https://doi.org/10.1016/j.jwpe.2025.108356 ↩ ↩2 ↩3 ↩4
-
Wang, X., Numedahl, N., & Jiang, C. (2024). Direct lithium extraction from Canadian oil and gas produced water using functional ionic liquids – A preliminary study. Applied Geochemistry, 172, 106126. https://doi.org/10.1016/j.apgeochem.2024.106126 ↩ ↩2
-
Karuppasamy, K., Mayyas, A., Alhseinat, E., Hassan-Beck, H., & Alfantazi, A. (2024). Exploring lithium extraction technologies in oil and gas field-produced waters: From waste to valuable resource. Chemical Engineering Journal Advances, 20, 100680. https://doi.org/10.1016/j.ceja.2024.100680 ↩ ↩2 ↩3
-
Farahbakhsh, J., Arshadi, F., Mofidi, Z., Mohseni-Dargah, M., Kök, C., Assefi, M., Soozanipour, A., Zargar, M., Asadnia, M., Boroumand, Y., Presser, V., & Razmjou, A. (2024). Direct lithium extraction: A new paradigm for lithium production and resource utilization. Desalination, 575, 117249. https://doi.org/10.1016/j.desal.2023.117249 ↩
-
Shan, Q., Zhu, G., Fan, P., Liang, M., Zhang, X., Liu, J., & Wu, G. (2025). Influence Mechanism of Coexisting Ions on the Extraction Efficiency of Lithium from Oil and Gas Field Water. Water, 17(15). https://doi.org/10.3390/w17152258 ↩ ↩2
-
https://www.aer.ca/regulations-and-compliance-enforcement/rules-and-regulations/directives/directive-051 ↩ ↩2 ↩3 ↩4
-
Muldoon, P. R., Williams, J., Lucas, A. R., Pickfield, P., & Gibson, R. B. (2020). An introduction to environmental law and policy in Canada (Third edition). Emond Montgomery Publications Limited. ↩
-
https://laws-lois.justice.gc.ca/eng/regulations/SOR-2001-286/page-33.html#h-1231646 ↩
-
https://laws-lois.justice.gc.ca/eng/regulations/sor-2001-286/page-31.html ↩